Heat Class 7 Notes Science Chapter 4
The energy that gives something its heat is called heat. In the winter, it's a common occurrence for us to feel cold inside the house and warm when we step outside into the sunlight. Now that we are aware of this, how do we experience warmth or cold? What will be our response, then? Think. We will attempt to answer this kind of question in this chapter.
Hot and Cold
Hot and cold are terms used to describe the temperature of an object or substance. "Hot" refers to high temperature, while "cold" refers to low temperature. These terms are relative, as what may be considered hot to one person may be considered cold to another.
Measuring Temperature
There are two kinds of thermometers:
Clinical thermometer: A device used to measure the temperature of the human body.
Laboratory thermometer: A thermometer used to measure the temperature of common objects.
Laboratory Thermometer
A laboratory thermometer is a tool used to gauge the temperature in a science-based environment.
This thermometer is constructed from a lengthy glass tube with a narrow bore.
The range of a laboratory thermometer is defined as the temperature range between -10°C and 110°C that can be measured using the graduations on the thermometer's tube
Reading a Laboratory Thermometer
The steps to reading a thermometer's reading are as follows.
Step I: Take some hot water and put it in a beaker first.
Step II: Attempt to grasp the glass tube of the lab thermometer now, and submerge the thermometer's bulb in the hot water in the beaker. Keep in mind that, as shown in the figure, the thermometer bulb should not touch either the sides or the bottom of the beaker.
Step III: We will now watch as the mercury thread in the thermometer tube rises. The temperature will eventually stop rising and remain stationary.
The flow of heat stops when the temperatures of the two objects are equal. This means that if the temperature of two objects is the same, no heat will be transferred between them.
Heat can be transferred from a hot object to a cold object in three different ways.
Using conduction (in solid, heat is transferred by conduction)
By tradition (in liquid and gases, heat is transferred by convection)
by radioactivity (in free space or vacuum, heat is transferred by radiation)
Let's talk about each of the three heat transfer mechanisms.
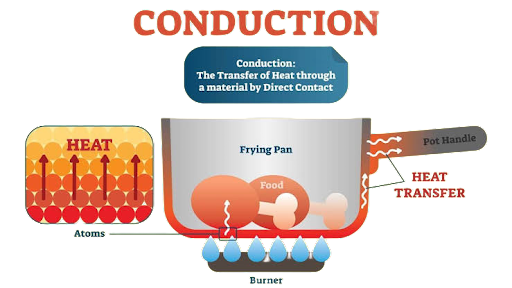
1. Conduction
Conduction is the process by which heat is transferred from a material's hotter to its cooler or from a hot material to a cold material in contact without the material moving as a whole. Conduction is the method used to transfer heat in all solids.
Heat Conductor and Insulator
Heat conductors are substances that permit easy heat conduction through them. Metals like iron, copper, silver, aluminum, etc., are effective heat conductors.
Materials that make it difficult for heat to pass through them are considered poor heat conductors. These substances are also referred to as heat insulators. Glass, plastic, and wood all act as heat insulators
Uses of Good and Bad Conductors of Heat
During the winter season, we usually wear woollen clothes, which have more space between them and are filled with air, which is a bad conductor of heat. Jute and sawdust are also bad conductors of heat, so we cover ice with a jute cloth of sawdust to prevent it from melting. The double walls of refrigerators have space inside which is filled with an insulating material, and two thinner blankets are very effective because the air layer trapped between the thinner blankets creates insulation and provides protection from cold.
When two things are at the same temperature, it can seem like they are at different temperatures. This is because some things are good conductors of heat, while others are poor conductors. For example, a metal object in a room feels cold to touch, while a wooden object in the same room feels warmer to touch. Water and air are bad conductors of heat, as they do not allow the heat of the hand to escape and thus feel warmer to touch.
2. Convection
Convection is the transfer of heat from the hotter part of a fluid (liquid or gas) to its colder parts by the movement of the liquid or gas itself. It is only possible in liquids and gases due to the free movement of particles.
Convection in Water
Air is a poor conductor of heat, transferring heat from hotter parts to colder parts through convection.
Sea and Land Breezes
The convection of heat in air causes the blowing of sea and land breezes in coastal areas.
Sea and land breezes are convection of heat, flowing from the sea towards the land during the day and blowing from the land towards the sea at night.
The land heats up more than water, causing the air over the land to become hotter and lighter, leading to a sea breeze. This sea breeze blows during the day due to the cooler and heavier air from the sea.
The air over the land warms up and becomes lighter because the land absorbs more heat than the water, which results in a sea breeze. Due to the cooler and denser air coming from the sea, this sea breeze blows during the day.
3. Radiation
The mode of transfer of heat from a hot body to a cold body by means of heat rays without any material medium between them is known as radiation. This is because the sun emits invisible heat radiation (or infrared rays) in all directions and travels through the vacuum between the sun and the earth at a very high speed, reaching us on the earth. Therefore, the transfer of heat from a hot object to a cold object by the process of radiation does not require any medium.
There are numerous instances in daily life where heat is transferred by radiation through the air, for example.
Our body radiates heat into the environment or absorbs heat from the environment depending on the temperature of the surrounding area.
A hot object filled with hot milk that is kept away from the flame cools down by radiating heat into the environment.
We will experience the heat of the fire falling on our faces if we are standing close to a burning fire. Radiation is the mechanism by which the heat from the fire is transferred to our faces.
Clothes
During hot summer days, people prefer to wear white or light coloured clothes to absorb less heat from the sun. In cold winter days, people prefer to wear dark clothes to absorb more heat rays from the sun and keep them warm. Woollen clothes are a poor conductor of heat due to the air trapped between the wool fibres, which prevents the flow of heat from the body to the cold surroundings, making them feel warm.







Hello,
May I help you ?